Why NanoZoomer
Products
Solutions
Case study
Resources
Japan (EN)
Select your region or country.
Eight markers, multiplex immunofluorescent staining
Maddalena M Bolognesi and Giorgio Cattoretti, Department of Pathology, Università di Milano-Bicocca, Milan, Italy.
Today’s pathology practice calls for an ever-shrinking size of tissue samples and an expanding need for immunostains. Fine needle biopsies are becoming the norm for diagnosis, staging and therapy. Cell block preparation is required to optimize the cytological diagnosis from fine needle aspirations. In solid tumors, such as lung cancer, there is a need to balance the necessity to save precious material for extractive molecular tests (EGFR) and the assessment of in-situ protein targets for advanced, personalized therapy aimed at activated oncogenes (ALK, BRAF, ROS), checkpoint inhibitors (PD-1, VISTA, PD-L1), etc. The cancer-driven impulse at immunomodulatory therapy has already impacted on the evaluation of biopsies obtained for autoimmune, inflammatory conditions such as systemic lupus erythematosus (SLE), chronic liver, intestinal and skin diseases, transplant rejection etc. These biopsies are traditionally minute, often unique tissue samples. Single cell analysis by cell suspension is not feasible and a relatively limited number of sections can be obtained in expert hands. Furthermore, formalin-fixed, paraffin-embedded (FFPE) material is almost invariably the only tissue available.
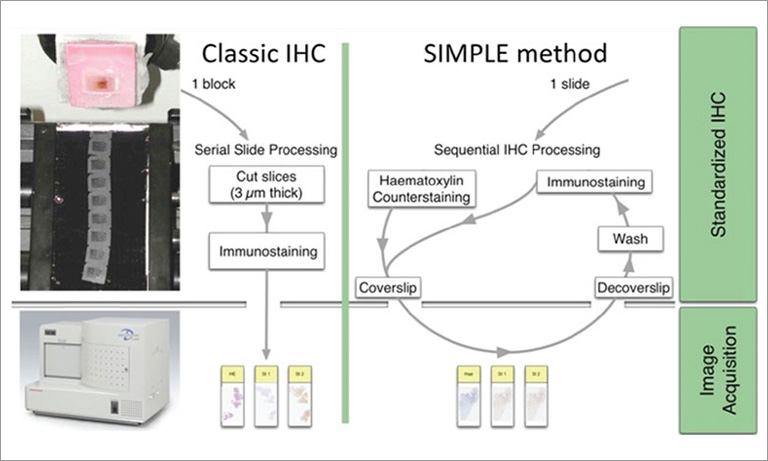
A solution to overcome these constraints is to do multiple stains on the very same section. Current protocols in IHC allow two-three stains on nonoverlapping cellular/subcellular targets [1]. There are more choices by using immunofluorescence (IF) stains.
Most IF microscopes are equipped with three IF channels, conventionally named after the prototype fluorochrome DAPI, FITC, TRITC. A fourth channel may be added, Cy5. Traditional microscopes however do not record whole slide images (WSI), limiting the documentation to selected fields, after which the fluorescence on the section fades out and no additional fields can be examined. On the contrary, IF slide scanners record the WSI in multiple channels, allowing a dynamic, complete and retrospective evaluation of any tissue area of choice. In 2015, Hamamatsu Photonics introduced the NanoZoomerⓇ S60 Digital slide scanner brightfield and IF scanner, which can accommodate up to 60 slides. The NanoZoomer S60 Digital slide scanner has two six-filter wheels, one for excitation, the other for emission filters, a threecube turret and is equipped with a Plan Apochromat Lambda 20x NA 0.75. Objective (Nikon), a Fluorescence Imaging Module L13820 equipped with a mercury lamp (Hamamatsu Photonics), and a ORCAⓇ-Flash 4.0 digital CMOS camera (Hamamatsu Photonics).
Choices for multiple IF stains are complicated by several limitations:
- Spectral unmixing of multiple excitation/emission wavelengths within the 350-750 nm span [2] requires ad hoc spectral IF microscopy apparatuses and software and can accomodate no more than 7 colors (including DAPI)
- DNA-barcode or directly fluorochrome conjugated antibody applications [3-5] require custom antibody conjugation, dedicated high NA, high sensitivity optics, multiple cycles of staining and quenching with only two fluorochromes at a time.
- Ion-tagged custom antibodies and in situ MALDI-type instruments can accomodate ~40 markers at 1µm/pixel resolution with a high cost of hardware investment [6].
Most of these techniques i) allow the staining of a single section per round, ii) do not support WSI, iii) reagents and instruments are so costly and timeconsuming that staining of multiple single sections is discouraged, or one or all of these combined. Another property of FFPE material is tissue autofluorescence (AF), which has restrained the application of IF for diagnosis or research.
A method to sequentially stain and strip an FFPE routinely processed section has been published [7]. This method employs widely available primary unconjugated and secondary IF antibodies, double indirect IF staining and digital tissue AF subtraction [7]. By carefully selecting primary antibodies produced in various species and/or of different immunoglobulin isotypes (e.g. Rabbit Ig + Mouse IgG1 + IgG2a/b + IgG3 or Rabbit + Mouse + Rat + Goat Ig) the full extent of the IF span of the NanoZoomer S60 Digital slide scanner can be exploited.
Four primary antibodies from one of the above mentioned selections can be visualized and acquired, in addition to the acquisition of the DAPI nuclear counterstain and the tissue AF for background subtraction. One single FFPE section is all that is required, different from a spectral unmixing acquisition, where for every desired wavelength a corresponding section is necessary for spectral identification and AF subtraction. To attain this goal, the standard NanoZoomer S60 Digital slide scanner setting was modified to accommodate DAPI, BV480, FITC, TRITC, Cy5 and AF, as shown in Figure 1.
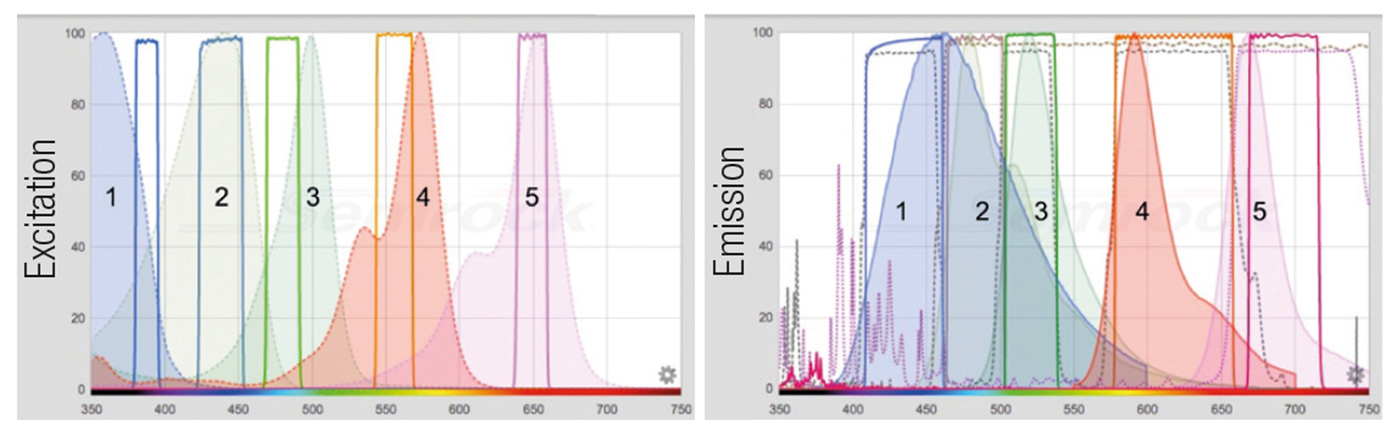
Figure 1: The composite panel represents the excitation filters and fluorochrome spectra (left) and the emission filters, dichroic mirrors and fluorochrome spectra (right). Excitation spectra are represented by a dashed profile, emission spectra by a solid profile. The filter profiles are represented by solid lines, the dichroic ones by a dashed line. 1: DAPI (359/461) [exc/em]; 2: BV480 (437/478); 3: Alexa 488 (499/519); 4: Rhodamine RedX (570/590); 5: Alexa 647 (652/668).
The filter combination depicted are DAPI: 387/11 – 435/40 [exc/em], BV480: 438/24 – 483/32; FITC: 480/17 – 520/28; TRITC: 556/20 – 617/73; Cy5: 650/13 – 694/44; AF: 438/24 – 617/73. The dichroic mirrors are: FF403/497/574-Di01 (triband), 458-Di02 and FF655-Di01.
Alexa® dyes are a Life Technologies patent. BV480 dye is a BD Biosciences patent. The spectra images are obtained with the Searchlight Semrock web application.
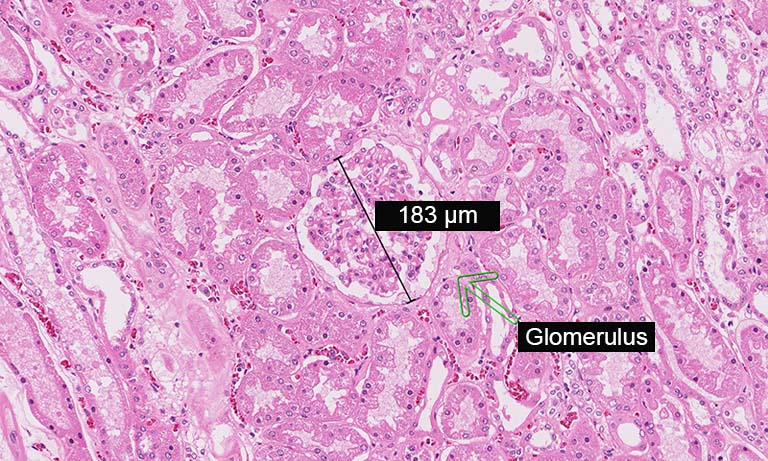
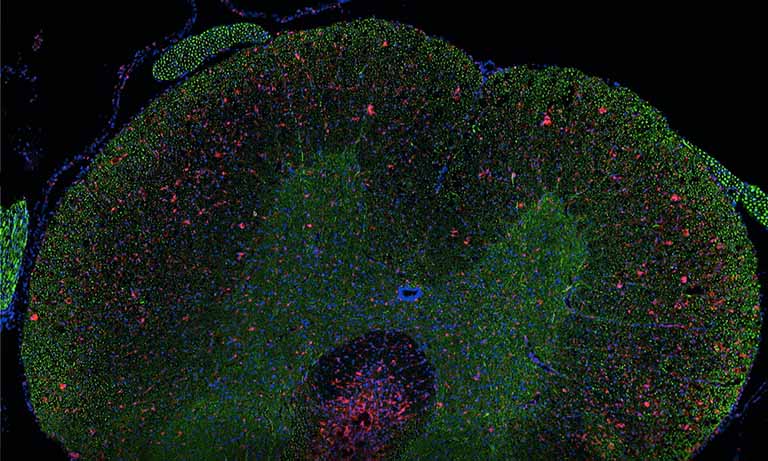
To address the immune environment and the tissue architecture of SLE nephritis on FFPE renal biopsies, we set up an 8-marker panel in IF, inclusive of T cells (CD3), B cells (CD20), monocyte/macrophages (CD68, CD163), plasmacytoid dendritic cells (pDC) (IRF8), endothelium (CD34), interstitial or glomerular fibroblasts (CD248) and interferon response genes (MX1). The antibody clone, the Ig specific and the secondary IF reagent are shown in Table 1. This panel was divided into two four-marker rounds, with one stripping cycle in between. For stripping we used the beta Mercaptoethanol/SDS protocol [7]; however, a friendlier protocol has been published [7], based on non-toxic compounds. CD34 and CD248 stain non-overlapping stromal components in the interstitium and define the fine architecture of the glomerulus (Fig 2). In the presence of an autoimmune inflammation, MX1 is induced upon interferon signaling.
The immune infiltrate, both inside and outside the glomerulus is defined in its components (T, B, pDC, Mono/Mac) and the distribution relative to each other and the kidney structure (Fig 2). A complete 8-marker panel can be accomplished in three day staining and stripping cycles; up to 40 individual cases can be processed for this multiplex panel in a working week. The ability to perform 8 established stainings, on multiple individual single sections, with a turnaround time compatible with clinical needs, brings this technique close to a clinical application of the multiplex IF staining with the NanoZoomer S60 Digital slide scanner. No other instrument/reagent combination available today can accomplish this task.
For research, sections stained with the 8-marker panel can be safely stored at -20 deg. C. in buffered glycerol [7] and sequentially stained and stripped in excess of 10 times (>44 markers), bringing this technique up to high-plex IF staining ([7] and Bolognesi MM et al, in preparation).
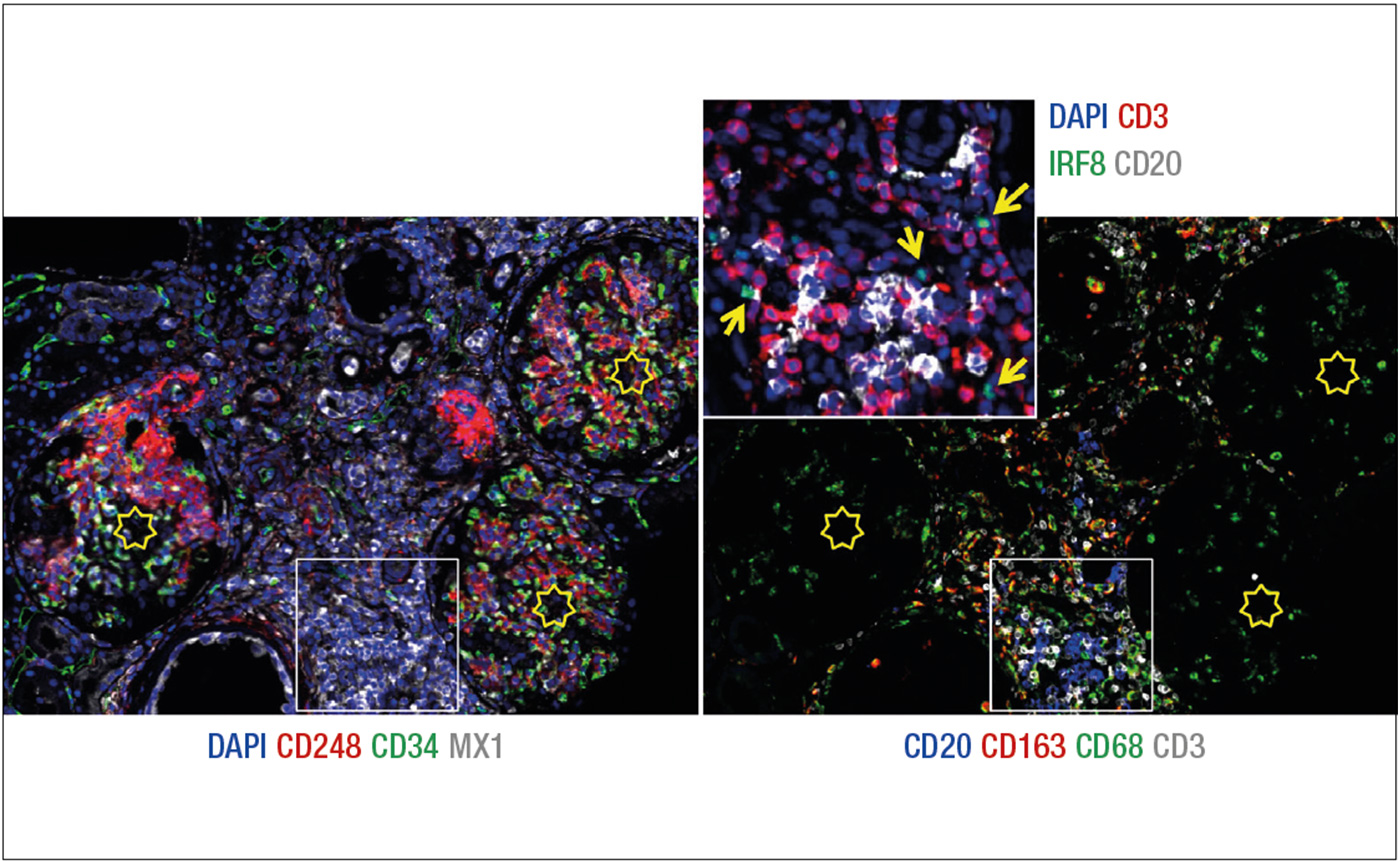
Figure 2: Eight markers (+DAPI) immunostaining of a renal biopsy with SLE. A selected field from a WSI is imaged as RGB+white composites.
Left: the image depicts three glomeruli (stars) containing CD248 mesangium (red) and CD34 endothelium (green). Sparse interstitial fibroblasts (red) and capillaries (green) are seen. MX1 (white) is induced in tubules, interstitial inflammatory cells and the endothelium of a vessel (lower center).
Right: the image shows macrophages (CD68, green) and histiocytes (CD163, red), admixed with CD20+ B cells (blue) and CD3+ T cells (white). CD68+ CD163- phagocytes predominate inside the glomeruli. The inflammation area in the white square, enlarged in the inset, shows IRF8+ plasmacytoid dendritic cells (green, arrowed) admixed with T cells (CD3, red) and B cells (CD20, white). DAPI (blue) counterstain. AF has been subtracted from individual grey level images before RGB merging.
Table 1
Primary and secondary antibodies used*
| Antibody |
Clone | Species | Isotype | Secondary Ab | Fluorochrome |
|---|---|---|---|---|---|
| CD3 | - | Rabbit | Ig | Gt a Rb | Rhodamine RedX |
| CD20 | L26 | Mouse | IgG2a | Gta Mo IgG2a | BV480 |
| CD163 | 10D6 | Mouse | IgG1 | Gt a Mo IgG1 | Alexa 488 |
| CD68 | PGM1 | Mouse | IgG3 | Gt a Mo IgG3 | Alexa 647 |
| MX1 | - | Rabbit | Ig | Gt a Rb | Alexa 488 |
| CD248 | B1/35 | Mouse | IgG1 | Gt a Mo IgG1 | Alexa 647 |
| IRF8 | E9 | Mouse | IgG2b | Gta Mo IgG2b | BV480 |
| CD34 | 3A1 | Mouse | IgG3 | Gt a Mo IgG3 | Rhodamine RedX |
*Secondary antibodies are from Jackson Immunoresearch
References
- W. A. Day et al., Covalently deposited dyes: a new chromogen paradigm that facilitates analysis of multiple biomarkers in situ. Lab Invest 97, 104-113 (2017).
- E. C. Stack, C. Wang, K. A. Roman, C. C. Hoyt, Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods, 70, 46-58 (2014).
- Y. Goltsev, N. Samusik, J. Kennedy-Darling, S. B. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. biorxiv.org, (2017) http://dx.doi.org/ 10.1101.
- W. Schubert et al., Imaging cycler microscopy. PNAS 111, E215 (2014).
- J.-R. Lin, M. Fallahi-Sichani, P. K. Sorger, Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nature Communications 6, 8390(2015).
- C. Giesen et al., Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nature methods 11, 417-422 (2014).
- M. M. Bolognesi et al., Multiplex Staining by Sequential Immunostaining and Antibody Removal on Routine Tissue Sections. Journal of Histochemistry & Cytochemistry 65, 431-444 (2017).
Eight markers, multiplex immunofluorescent staining [1.5 MB/PDF]

Other research case study
- Confirmation
-
It looks like you're in the . If this is not your location, please select the correct region or country below.
You're headed to Hamamatsu Photonics website for JP (English). If you want to view an other country's site, the optimized information will be provided by selecting options below.
In order to use this website comfortably, we use cookies. For cookie details please see our cookie policy.
- Cookie Policy
-
This website or its third-party tools use cookies, which are necessary to its functioning and required to achieve the purposes illustrated in this cookie policy. By closing the cookie warning banner, scrolling the page, clicking a link or continuing to browse otherwise, you agree to the use of cookies.
Hamamatsu uses cookies in order to enhance your experience on our website and ensure that our website functions.
You can visit this page at any time to learn more about cookies, get the most up to date information on how we use cookies and manage your cookie settings. We will not use cookies for any purpose other than the ones stated, but please note that we reserve the right to update our cookies.
1. What are cookies?
For modern websites to work according to visitor’s expectations, they need to collect certain basic information about visitors. To do this, a site will create small text files which are placed on visitor’s devices (computer or mobile) - these files are known as cookies when you access a website. Cookies are used in order to make websites function and work efficiently. Cookies are uniquely assigned to each visitor and can only be read by a web server in the domain that issued the cookie to the visitor. Cookies cannot be used to run programs or deliver viruses to a visitor’s device.
Cookies do various jobs which make the visitor’s experience of the internet much smoother and more interactive. For instance, cookies are used to remember the visitor’s preferences on sites they visit often, to remember language preference and to help navigate between pages more efficiently. Much, though not all, of the data collected is anonymous, though some of it is designed to detect browsing patterns and approximate geographical location to improve the visitor experience.
Certain type of cookies may require the data subject’s consent before storing them on the computer.
2. What are the different types of cookies?
This website uses two types of cookies:
- First party cookies. For our website, the first party cookies are controlled and maintained by Hamamatsu. No other parties have access to these cookies.
- Third party cookies. These cookies are implemented by organizations outside Hamamatsu. We do not have access to the data in these cookies, but we use these cookies to improve the overall website experience.
3. How do we use cookies?
This website uses cookies for following purposes:
- Certain cookies are necessary for our website to function. These are strictly necessary cookies and are required to enable website access, support navigation or provide relevant content. These cookies direct you to the correct region or country, and support security and ecommerce. Strictly necessary cookies also enforce your privacy preferences. Without these strictly necessary cookies, much of our website will not function.
- Analytics cookies are used to track website usage. This data enables us to improve our website usability, performance and website administration. In our analytics cookies, we do not store any personal identifying information.
- Functionality cookies. These are used to recognize you when you return to our website. This enables us to personalize our content for you, greet you by name and remember your preferences (for example, your choice of language or region).
- These cookies record your visit to our website, the pages you have visited and the links you have followed. We will use this information to make our website and the advertising displayed on it more relevant to your interests. We may also share this information with third parties for this purpose.
Cookies help us help you. Through the use of cookies, we learn what is important to our visitors and we develop and enhance website content and functionality to support your experience. Much of our website can be accessed if cookies are disabled, however certain website functions may not work. And, we believe your current and future visits will be enhanced if cookies are enabled.
4. Which cookies do we use?
There are two ways to manage cookie preferences.
- You can set your cookie preferences on your device or in your browser.
- You can set your cookie preferences at the website level.
If you don’t want to receive cookies, you can modify your browser so that it notifies you when cookies are sent to it or you can refuse cookies altogether. You can also delete cookies that have already been set.
If you wish to restrict or block web browser cookies which are set on your device then you can do this through your browser settings; the Help function within your browser should tell you how. Alternatively, you may wish to visit www.aboutcookies.org, which contains comprehensive information on how to do this on a wide variety of desktop browsers.
5. What are Internet tags and how do we use them with cookies?
Occasionally, we may use internet tags (also known as action tags, single-pixel GIFs, clear GIFs, invisible GIFs and 1-by-1 GIFs) at this site and may deploy these tags/cookies through a third-party advertising partner or a web analytical service partner which may be located and store the respective information (including your IP-address) in a foreign country. These tags/cookies are placed on both online advertisements that bring users to this site and on different pages of this site. We use this technology to measure the visitors' responses to our sites and the effectiveness of our advertising campaigns (including how many times a page is opened and which information is consulted) as well as to evaluate your use of this website. The third-party partner or the web analytical service partner may be able to collect data about visitors to our and other sites because of these internet tags/cookies, may compose reports regarding the website’s activity for us and may provide further services which are related to the use of the website and the internet. They may provide such information to other parties if there is a legal requirement that they do so, or if they hire the other parties to process information on their behalf.
If you would like more information about web tags and cookies associated with on-line advertising or to opt-out of third-party collection of this information, please visit the Network Advertising Initiative website http://www.networkadvertising.org.
6. Analytics and Advertisement Cookies
We use third-party cookies (such as Google Analytics) to track visitors on our website, to get reports about how visitors use the website and to inform, optimize and serve ads based on someone's past visits to our website.
You may opt-out of Google Analytics cookies by the websites provided by Google:
https://tools.google.com/dlpage/gaoptout?hl=en
As provided in this Privacy Policy (Article 5), you can learn more about opt-out cookies by the website provided by Network Advertising Initiative:
http://www.networkadvertising.org
We inform you that in such case you will not be able to wholly use all functions of our website.
Close